A Layman’s Guide to Navigate a Climate ‘Crisis’ - Part 5
Volume 4 / Special Issue / 5. Nominee No. 1 – Solar Radiation Management
Hello Again!
If you missed the previous post, 4. Public Enemy No. 2 – Fossil Fuels, the link below will take you to it to put things into context.
5. Nominee No. 1 – Solar Radiation Management
Quick overview - In the first part of the section, the contents focus specifically on SRM practices. In the second part of the section, we talk about air quality as well as the monitoring thereof, and the role that dust deposition plays. Lastly, we look at a case study.
As already established, increased amounts of CO2 is good for the overall health of the Earth. Consider then the implications of Greenhouse Gas Removal (GGR) and Solar Radiation Management (SRM).
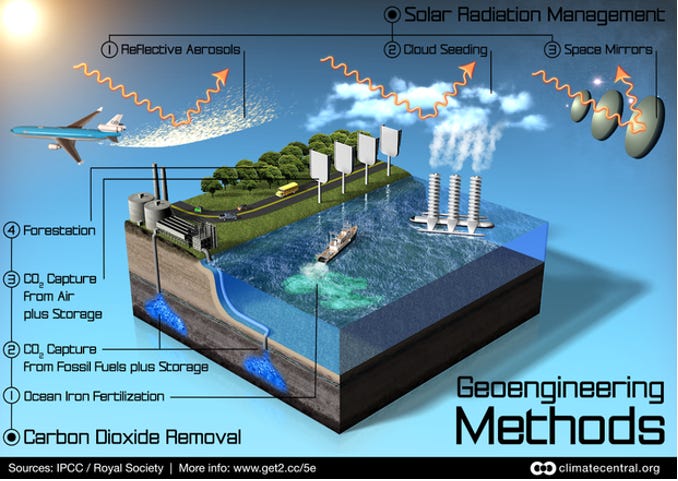
These terms describe a range of technologies that aim to counteract ‘human-caused’ climate change by deliberate large-scale intervention in the Earth’s natural systems. They are also referred to as ‘geo-engineering’ or ‘climate engineering’ or ‘albedo modification’. Examples of SRM technologies include the brightening of marine clouds and injection of aerosols into the stratosphere called Stratospheric Aerosol Injection (SAI).
The Carbon Brief (2018) listed six commonly proposed solar geoengineering technologies and included: aerosol injection, ocean mirror, marine cloud brightening, cloud thinning, high-albedo crops and buildings, and space sunshades.
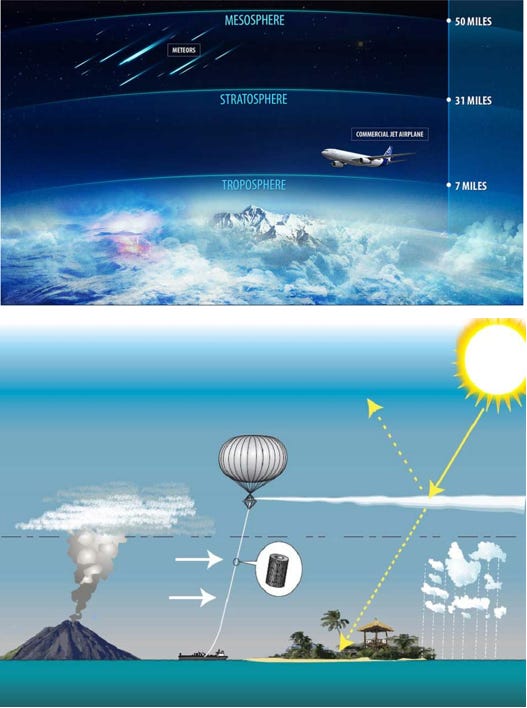
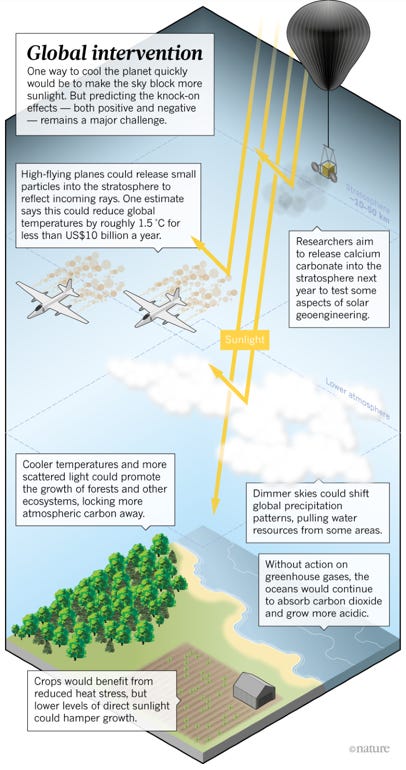
This technique (SAI) aims to reduce the incidence and absorption of the sun's rays by reflecting back a small portion of solar radiation. Various proposals seek to do this by increasing the earth's reflecting power, thus providing a cooling effect to mitigate the warming influence caused by an ever-increasing amount of greenhouse gases. This approach involves spraying reflective sulphate aerosol particles into the stratosphere with high altitude airplanes, tethered balloons, high-altitude blimps, or artillery.
5.1 Background and Theory of SRM
According to NASA, aerosols are tiny particles suspended in the atmosphere. When these particles are sufficiently large, we notice their presence as they scatter and absorb sunlight. Their scattering of sunlight can reduce visibility (misty haze) and redden sunrises and sunsets. Aerosols interact both directly and indirectly with the Earth's radiation budget and climate. They also affect human life on earth by transporting diseases, and nutrients across the globe. As a direct effect, the aerosols scatter sunlight directly back into space. As an indirect effect, aerosols in the lower atmosphere can modify the size of cloud particles, changing how the clouds reflect and absorb sunlight, thereby affecting the Earth's energy balance.
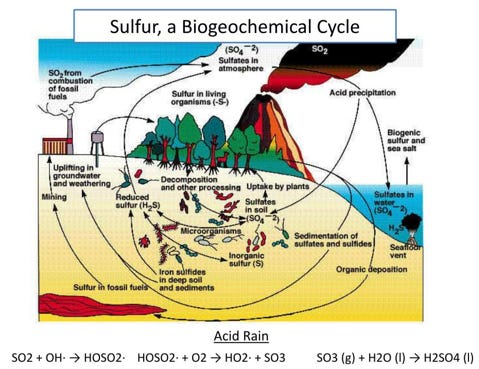
Stratospheric Aerosol Injection (SAI) is a theoretical solar geoengineering proposal to spray large quantities of tiny reflective particles into the stratosphere, to cool the Planet by reflecting sunlight back into space. It is considered relatively safe for the environment based on the premise that volcanoes have been injecting sulphate aerosols into the stratosphere for eons and the biosphere naturally processes sulphates using multiple pathways in the sulphur biogeochemical cycle.
This method would mimic what large volcanoes do. The Mount Pinatubo as an example was the largest eruption of the 20th century and injected 20 million tons of sulphur dioxide aerosols into the stratosphere where the Earth’s lower atmosphere temperature dropped by approximately 1.3-degree Fahrenheit (-17.2°C). The effect only lasted a couple of years because the sulphates eventually fell to Earth.
Most stratospheric aerosols are formulated in the troposphere where they are transported by wind or sedimentation and eventually reach the stratosphere by rapidly mixing, mainly through weather events or turbulence. According to the National Academy of Science's study, aerosols in the upper atmosphere take longer to remove because they are located away from most clouds and typical weather phenomena which would remove and recycle these aerosols within days, making the SAI method even more feasible. Models and theory suggest that increasing the number of aerosols in the atmosphere would cool the earth, mitigating some of the effects of greenhouse gases on our planet.
Much of the stratospheric sulphate aerosols (i.e., sulphur dioxide (SO2), carbonyl sulphide (COS), and sulfuric acid (H2SO4)) are formed through both natural and anthropogenic processes originally from the Earth's surface. By adding oxygen atoms to these gases through a series of chemical reactions, eventually leads to the formation of sulphate aerosols.
According to the Environmental Protection Agency (EPA), the largest source of SO2 in the atmosphere is from the burning of fossil fuels by power plants and other industrial facilities. Smaller sources of SO2 emissions include natural sources such as volcanoes, industrial processes such as extracting metal from ore, locomotives, ships and other vehicles and heavy equipment that burn fuel with a high sulphur content. Furthermore, there is a very small concentration of about 1 ppm (parts per million) in the atmosphere, where it reacts with water to form clouds of sulfuric acid, which is key component of the Plant’s global atmospheric sulphur cycle and is also a contributor to global warming.
In theory, Stratospheric Aerosol Injection (SAI) is feasible with existing technologies. It could also be implemented in a very short period of time and at a relatively low cost. Because stratospheric aerosols (larger particles) naturally fall out of the atmosphere, the benefits/effects of this approach, are temporary, generally lasting between 1 to 2 years.
The official narrative is that SRM or SAI has not been deployed yet, and that as recent as 2022 new funding and grants became available to continue with geoengineering research, which will include efforts to assess climate interventions.
5.2 Publicly Documented Experiments & Claims
The only known field experiments that have been carried out in the open air was by the University of California, San Diego, where researchers sprayed smoke and salt particles off the coast of California as part of the E-PEACE experiment in 2011, and scientists in Russia dispersed sulphate aerosols from a helicopter and car in 2009. The so-called SPICE experiment in the United Kingdom was quickly scuttled in 2012, following public criticism and conflict of interest accusations after several of the scientists applied for a related patent.
Alongside other geoengineers and researchers, David Keith, founder of Harvard’s Solar Geoengineering Research Program, and author of the book A Case for Climate Engineering (2013), proposed a number of field experiments in March 2017 (The Guardian) including the “Stratospheric Controlled Perturbation Experiment” (SCoPEx). The aim of this experiment was to acquire further data for modelling solar geoengineering and predicting larger-scale effects of SAI, by monitoring the reflective properties of injected particles and their impact on the surrounding atmosphere. The envisaged field test aimed to disperse various particles of different chemicals (sulphur dioxide, alumina, or calcium carbonate), from a balloon into the stratosphere, at an altitude of 20 kilometres above the Earth. The proposal envisaged that the balloon will be steered from ground and equipped with scientific instruments, including sensors for data collection.
It was also reported in another article, also in March 2017 (MIT Technology Review), that future tests could involve seeding the sky with aluminium oxide – or even diamonds.
David Keith was quoted to state the following at seminars and in public interviews since 2011:
“It’s about the fact, and it’s an uncomfortable fact, but it is a fact that we have the technical ability to do this. They are fast acting; they are cheap, and they are fundamentally imperfect.
So, if there was a decision to do a geoengineering program and you put about a million tons per year you might end up killing many tens of thousands of people per year as a direct result of that decision.
The problem is the following: If you add SO2 to the stratosphere, SO2 doesn’t condense so you might want to put alumina in. Alumina has a very high refraction, it is very small, it doesn’t coagulate, and you can engineer the particles with particular properties.
Smaller sized particles mean more surface area, but more surface area means less ozone.”
5.3 Uncertainties and Impacts of the Technology
The UN’s intergovernmental panel on climate change, said in March 2017 that “Cutting incoming solar radiation affects the weather and hydrological cycle. It promotes drought. It destabilizes things and could cause wars. The side effects are many and our models are just not good enough to predict the outcomes. A 1991 Mount Pinatubo eruption lowered global temperatures by 0.5°C, while the Mount Tambora eruption in 1815 triggered Europe’s ‘year without a summer’, bringing crop failure, famine, and disease”.
In 2018, the American Meteorological Society (AMS) expressed concerns that the possibility of seemingly quick and inexpensive fixes will distract the public and policymakers from addressing the underlying problems and developing adaptation strategies. The AMS official policy statement regarding this type of geoengineering stated: "Reflecting sunlight would likely reduce Earth's average temperature but could also change global circulation patterns with potentially serious consequences such as changing storm tracks and precipitation patterns".
According to Conant et al. (2002), atmospheric aerosols reduce the solar radiation absorbed by the Earth through direct and indirect mechanisms. In the direct effect, aerosols scatter radiation to space that would have otherwise heated the surface or atmosphere. In the indirect effect, increased concentrations of cloud condensation nuclei (CCN) alter the microphysical properties of clouds, enhancing cloud reflectivity. Additional indirect effects on clouds involve aerosol-induced changes in precipitation, radiative heating, and atmospheric dynamics. In contrast, absorptive particles, black carbon (e.g., soot) in particular, exert a positive (warming) climate forcing.
“Black carbon plays a role in indirect aerosol radiative forcing in two ways: 1) BC particles absorb solar radiation, changing the vertical and horizontal thermal structure of the atmosphere and surface, changes that can have an impact on dynamical processes governing cloud formation and evaporation; 2) Those particles that act as CCN and become incorporated in cloud droplets absorb solar radiation and heat the droplets and the air around them, processes that can potentially alter cloud activation itself” (Conant et al., 2002). The uncertainties in distribution of natural and anthropogenic aerosols, their chemical speciation, and the aerosol-cloud-climate interactions constitute the largest source of uncertainty in climate models.
In other words, the systems in the Earth’s atmosphere are complex. Any quick fixes are bound to have unintended consequences and possibly cause a new set of problems. The AMS went on stating that the results of reflecting sunlight "would almost certainly not be the same for all nations and peoples, thus raising legal, ethical, diplomatic and national security concerns”. “One region may become a desert, while others become flooded out” (AMS,2018).
As with all solar geoengineering technologies that only address global surface temperatures, dramatic agitations in the climate system can be expected if SAI is deployed. SAI would modify the Earth’s radiative balance and is also associated with significant potential risks and uncertainties, such as impacts on extreme weather events, ecosystems, rain patterns, agricultural yields, ozone chemistry, solar energy output, human health and many more.
The following lists potential problems and risks by geoengineering practices:
Ozone depletion - which would reduce our protection from radiation from sunlight (UV rays)
Whitening of the sky: There would be an effect on the appearance of the sky from stratospheric aerosol injection, notably a slight hazing of blue skies and a change in the appearance of sunsets.
Stratospheric temperature change: Aerosols can also absorb some radiation from the Sun, the Earth, and the surrounding atmosphere. This changes the surrounding air temperature and could potentially impact the stratospheric circulation, which in turn may impact the surface circulation.
Deposition and acid rain: The surface deposition of sulphate injected into the stratosphere may also have an impact on ecosystems.
Ecological consequences: The consequences of stratospheric aerosol injection on ecological systems are unknown.
Effects on agriculture: Mixed effects on global crop yields of certain major crops.
Early research into SAI from the UK’s Met Office Hadley Centre found that SAI could lead to severe drought in the Sahel region of Africa. While researchers found that this could possibly be countered by injecting particles into the southern hemisphere’s stratosphere instead, this would likely cause a failure of the rains in northeast Brazil.
A modelling study (2020) found that injection in the northern hemisphere would lead to fewer hurricanes in the North Atlantic, which might be good news for the Caribbean, but it would likely create drought in Sub-Saharan Africa and parts of India. Injecting aerosols in the southern hemisphere wouldn’t create drought, but it would create more hurricanes in the North Atlantic. Regional warming is also likely, based on the results of the Geoengineering Model Intercomparison Project published in 2014. It predicted that temperatures in the tropics would cool as a result of SAI, but higher latitudes would warm.
A scientific study published in Nature in 2018 stated that the so-called ‘termination effect’ is another major risk and quoted: “If geoengineering were halted all at once, there would be rapid temperature and precipitation increases at 5–10 times the rates from gradual global warming.” The study also showed that the shock caused by sudden termination of solar geoengineering would have grave impacts on biodiversity. This means that stopping SAI once it had started could be more dangerous than starting it in the first place.
Studies on the impacts of SAI on public health are limited, but a recent analysis suggests that adverse public health impacts may be expected. There are also very few means of evaluating potential public health impacts should SAI ever be deployed.
5.4 Known Particle Substances Used for SAI
Sulphur Dioxide (SO2) – Can affect both human health and the environment. Short-term exposures to SO2 can harm the human respiratory system and make breathing difficult. At high concentrations, gaseous SO2 can harm trees and plants by damaging foliage and stunt growth.
Sulphur is a non-metal. Sulphide occurs naturally in mineral ores, oil, and coal deposits, and from the natural anaerobic decomposition of organic matter containing sulphur or sulphur-containing compounds. Sulphur is a common acidifying material, ultimately altering the acid-base equilibria in natural waters and soils and result in a reduced acid neutralising capacity, and hence, lowering of the pH.
Sulphur dioxide (SO2) dissolves easily in water. Atmospheric sulphur dioxide (SO2), discharged on combustion of fossil fuels, can give rise to sulphuric acid in rainwater (acid-rain) and as such, this results in the return of sulphate to the environment.
SO2, with its specific smell, is the most dominant gas associated with air pollution emitted by oil refineries (Al-Jahdali and Bisher, 2008). Accumulated SO2 levels in soils changes its pH causing acidity and thereby, affecting soil fertility (Al-Jahdali and Bisher, 2008).
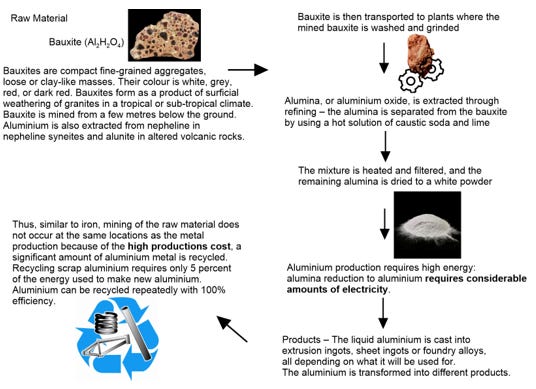
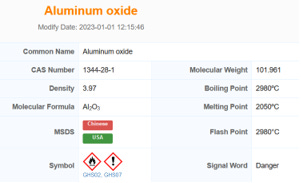
Aluminium oxide (Al2O3) – is a chemical compound of aluminium and oxygen. It is commonly referred to as Alumina. Aluminium oxide is responsible for the resistance of metallic aluminium to weathering. Aluminium oxide is insoluble in water.
Below is a quick and dirty guide on how Aluminium is made.
Aluminium oxide is on the EPA's Toxics Release Inventory list.
The oxidation of the surface of some liquid metal alloys leads to the formation of loose or porous 3D nanostructures. The nanofibers of such kind do not occur in nature and only produced by artificial means.
The applications of alumina nano fibres include:
Adsorbent (adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface)
Desiccant (a moisture absorbing substance used as a drying agent)
Sorbent (material used to absorb or adsorb liquids or gases)
Ceramics and composites (including composite metals) — high toughness, fire resistance and anti-friction properties, insulating properties.
Abrasive (composed of means for ultra-fine polishing)
Refractory (a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures, therefore a high-temperature component for heat insulation)
What happens when Aluminium enters the environment?
Aluminium cannot be destroyed in the environment; it can only change its form.
In the air, aluminium binds to small particles, which can stay suspended for many days.
Under most conditions, a small amount of aluminium will dissolve in lakes, streams, and rivers.
It can be taken up by some plants from soil.
Exposure in humans to high levels of aluminium may result in respiratory and neurological problems and tumours. A published article in Pubmed (2014) stated that Aluminium is neurotoxic. Its free ion, Al3+(aqueous), is highly biologically reactive and uniquely equipped to do damage to essential cellular (neuronal) biochemistry. Aluminium is present in the human brain, and it accumulates with age.
By injecting 10 – 20 million tons of Nanomaterials on top of industrialised pollution – what would this mean for the mortality of species? Also, considering: If we have Greenhouse gasses (and others) rising from the surface with a blanket of sun reflecting nanoparticles in top layers of our atmosphere – Are you not then trapping the GHGs and the CO2 and thereby heating the Planet even further?
5.5 The Ethical Conundrum Regarding SAI
Three ethical questions that should be asked when undertaking research and considering the use of geoengineering and stratospheric aerosol injection regarding climate mitigation issues. Would deliberate geoengineering be unethical and are some geoengineering techniques more ethically acceptable than others - if so, which and why?
Is a higher standard of proof or confidence needed for geoengineering interventions than for other mitigation actions?
What are the main ethical considerations that the design of a regulatory framework for geoengineering research or deployment would need to take into account?
Because of the unequal global impacts and its potential to be weaponized, solar geoengineering carries insurmountable challenges for governance. The National Academy of Science devised the following three questions:
Who decides if the benefits of albedo modification outweigh the risks, and what are the criteria?
Who gets to decide when and in what way albedo modification will be undertaken?
Would society ever know enough to responsibly decide to deploy albedo modification?
To recap: SRM technologies aim to inject more SO2 particulates into the stratosphere on a more regular basis in order to maintain concentrations high enough to create an artificial cooling effect on the Earth, whilst current air quality, in South Africa alone have ‘Very Unhealthy’ air quality with both health and environmental impacts.
5.6 Limitations of SAI
Christoph Kleinschmitt (2015) from the Institute of Environmental Physics, expressed that there are numerous risks and undesirable side effects of SAI, but they largely rely on the assumption that increasing radiative forcing by greenhouse gasses can be counterbalanced by sufficiently large aerosol injections, at least on a global scale. He stated that there are various processes that have the potential to severely limit the efficacy of SAI which are not considered in most models. These include coagulation of aerosol particles leading to an increase in particle size, which reduces scattering efficiency and stratospheric lifetime (in other words, they disperse quickly), and interaction with infrared radiation, i.e., the aerosol’s own greenhouse effect which can limit the desired cooling effect.
Robock (2008) noted that changing the global temperatures through SRM practices without lowering the level of greenhouse gas concentrations is of much concern and that a risk of lessening rainfall is a possibility. There could also be a weakening of the Indian or African monsoons, which is a particular worry.
5.7 Up, Up and Away
5.7.1 Air Quality Monitoring
Most air pollutants actively monitored in the UK are Ammonia (NH4), Carbon monoxide (CO), Nitrogen oxides (NOx), Non-Methane Volatile Organic Compounds (NMVOCs), Particulate matter (PM2.5 & PM10), Sulphur dioxide (SO2) and Lead (Pb). Similarly, in accordance with the SANS Ambient air quality – Limits for common pollutants lists Sulphur dioxide (SO2), Nitrogen dioxide (NO2), Carbon monoxide (CO), Particulate matter (PM2.5 & PM10), Ozone (O3), Lead (Pb), Benzene (C6H6) and Dust deposition for mandatory monitoring.
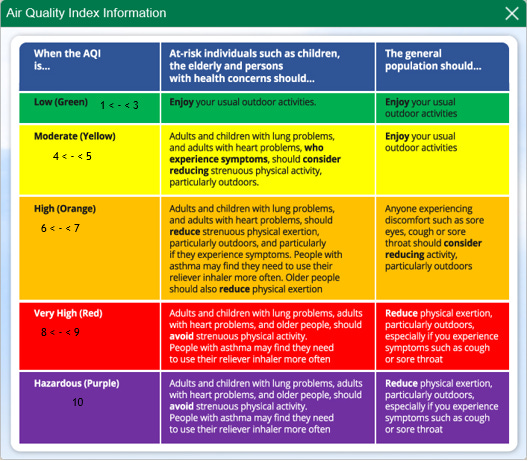
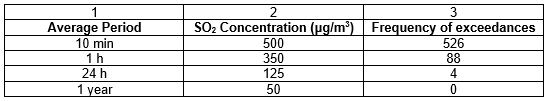
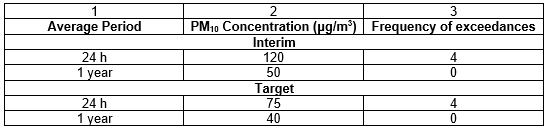
The South African Air Quality Information System (SAAQIS) provides a common platform where access to air quality information is available at stations across South Africa. Each station provides averaged hourly readings with optional viewing of weekly or monthly data. Though, only Sulphur Dioxide (SO2) and PM10 (particulate matter) are the components actively monitored as most of the stations only has available data for those 2 parameters. Below is the official legend and explanation of what the index used on the website means.
PM10 (Particulate matter 10) – Particulate matter (PM) is everything in the air that is not a gas and therefore consists of a huge variety of chemical compounds and materials, some of which can be toxic. Exposure to PM can result in serious impacts to health, and those with respiratory problems. As a result, particulates are classified according to size: particles of less than 10 micrometres in diameter (PM10) and less than 2.5 micrometres in diameter (PM2.5) is the most measured. Both PMs and the precursor pollutants that can form, travel large distances in the atmosphere. PM10 particles include fumes, fuels, smoke, pollen, sea salt and dust to name a few.
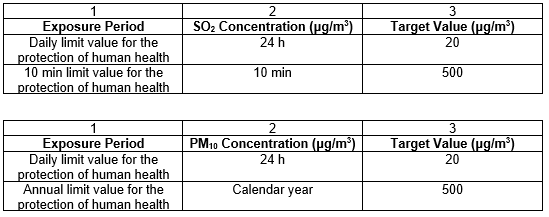
The SANS 1929:2011 detailed standards for exposure to SO2 and PM10, respectively and are summarised in the Tables below.
The SANS further define target levels which is described as the ambient air quality levels that provide an adequate development buffer between air that is considered harmful and air that is not considered harmful to health and wellbeing.
With this, consider the daily exposure levels for prolonged periods and the effect thereof on long term human health.
5.7.2 Dust deposition
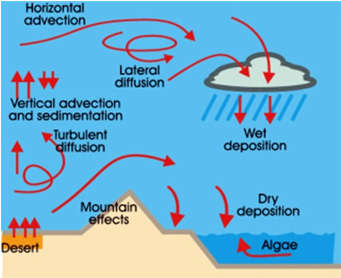
The dust in the atmosphere originates from different sources including, but not limited to, sea salts, fine soil, smoke-soot, ash, pollen, dust, and disintegrated particles of meteors. Other sources of dust include wood burning, coal and oil combustion, coal combustion emissions plus photochemical sulphate production, metal production plus municipal waste incineration, emissions from motor vehicles, and emissions from smelting of nonferrous metal ores and arsenic. Dust particles are generally concentrated in the lower layers of the atmosphere; however, convectional air currents may transport them to great heights. Higher concentrations of dust particles are found in the subtropical and temperate regions due to dry winds in comparison to equatorial and Polar Regions.
Dust and salt particles act as hygroscopic nuclei ((microscopic particle (e.g., of sulphur dioxide, salt, dust, or smoke) in the free air) around which water vapor condenses to produce clouds. The dust found in the atmosphere is made from rock and mineral particles, pollen, sea salt, chemicals, and bacteria. These dust particles cause hazy skies and provide a surface for condensation of water.
One particle of dust is so small that it is very difficult to see. When the sunlight in a room is just right, then one can observe some specs of dust hanging about and it is usually a lot more than expected.
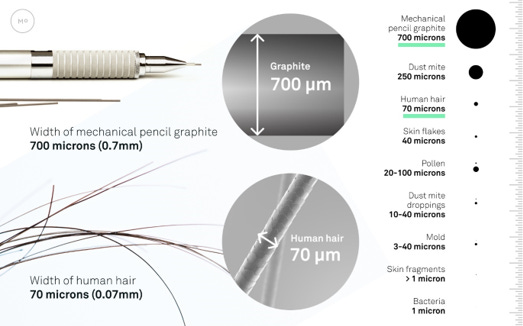
Particles that are too small to see usually do the most damage because they are breathed in. Coarser particles above about 10 microns (like PM10), or about 1/7th the width of a human hair usually affect the upper respiratory tract, finer particles of 2.5 micron (PM2.5) will affect the lower respiratory tract. Particles that are 0.1 micron and smaller may enter the bloodstream and have been linked to negative health effects throughout the human body.
Zhang, G., Ding, C., Jiang, X. et al. (2020) looked at the chemical compositions and source contribution of atmospheric particles at a typical steel industrial urban site in Laiwu, China by monitoring the PM2.5 concentrations.
The focus of the paper was on PM pollution sources, including soil dust, urban dust, construction dust, coal-fired power plants dust, steel plant dust and motor vehicle exhaust dust, and these were sampled, respectively. The authors chose a less urbanized city in China as the case study as there were very few detailed studies done on the topic. They argue that localised studies are necessary as regional atmospheric environments are interconnected, and that the ambient air in one city can affect the air quality in other cities via meteorological transport processes.
An excerpt from the paper: “It is commonly known that PM pollution is associated with poor atmospheric visibility, high health risks, and global climate change, and light extinction. The chemical compounds of PM contain ionic species, carbonaceous species, and metals and metalloids. Secondary inorganic species, such as NO3−, SO42−, and NH4+, which are water-soluble ions, are also known to be present in PM2.5 and can comprise a large fraction of PM in the atmosphere. Organic carbon (OC), composed of thousands of organic compounds, originates from both natural and artificial sources. Element carbon (EC), with relatively stable chemical properties, is directly emitted from primary combustion and influences the global climate system through its impact on radiative forcing”.
Note: Radiative forcing is what happens when the amount of energy that enters the Earth’s atmosphere is different from the amount of energy that leaves it.
The sampling results indicated that the characteristic components were silica (Si), iron (Fe) and calcium (Ca) in urban dust and soil dust; Ca, magnesium (Mg) and ammonia (NH4+) in construction dust; Fe, Ca and sulphate (SO42−) in steel dust; organic carbon (OC), elemental carbon (EC) and Si in motor vehicle exhaust dust; SO42−, aluminium (Al) and NH4+ in power plant dust, respectively.
The Chemical Mass Balance (CMB) model results showed that the contribution rates of the primary pollution sources apportionment for power plants dust and steel dust were 11% and 6%, and the annual contribution rate of secondary generation sources including sulphate, nitrate, and SOC to PM2.5 was 17%, 17% and 13%, respectively. Therefore, more attentions should be paid on the secondary sources than from the primary emission sources such as the motor vehicle exhaust, and coal combustion sources especially.
In 2009, the USGS noted that different minerals in dust will have different effects on the snow albedo. Iron oxides (Fe3+ in its oxidative state such as hematite and goethite) is a strong absorber of solar radiation and can thereby warm when exposed to sunlight, and this was observed when these particles heated up surrounding snow crystals exhibiting a ‘black box’ effect. They further argued that there are connections between iron oxide minerals and the radiative properties of dust.
5.8 Social Media Blow-Up – The Case of the Tyre Graveyard
In an article of the Observers of France24 dated August 2021 reported that the world’s largest tyre graveyard namely Sulaibiya tyre graveyard, located in Kuwait’s Jahra district, caught fire, in April 2021, and clouds of thick black smoke was filmed entering the atmosphere. It was reported that firefighters managed to put out the flames after 7 hours and the cause of the fire was arson.
This tyre landfill extends over 3 km2 and is considered the world's largest tyre graveyard where there are reportedly 60 million used tyres in the landfill alone, and according to environmental officials, will eventually be buried in large pits. In the 1980s and 1990s, Kuwait made a business importing used tyres from other countries, importing 259 million used tyres per year from the United States and Europe, until the practice was banned in 2001.
These tyre graveyards contain thousands of tonnes of rubber, which are exposed to the burning sun, thus releasing carcinogenic black carbon gas harmful to humans and the environment. Dioxins are released into the atmosphere when synthetic rubber combusts, even at low temperatures.
Think About It…
The “hazing” effect NASA referred to with the use of the SAI technology, what does it then mean for Solar power uptake to those countries that rely on it for power generation, like South Africa?
Scientists reported that the world might be cooler by implementing SRM technologies, but blue skies would become less blue, even in remote areas, under a whitish sky resembling the haze that blankets most major cities.
Will solar power generation be sustainable?
Many incidents of environmental contamination occurs much more often than it should. Be it, the case of the tyre graveyard contributing to air pollution and damaging environmental integrity or from petrol/diesel/chemical spills via tanker or rail accidents or leaks from underground infrastructure or from pollution to critical water resources via mining practices.
Point being, there are many incidents that do not make front page news to drum up outrage, however, these are directly hazardous not only to human health, but also to the overall health of the surrounding environment, which includes water resources, fauna, and flora.
Coming Soon - 6. Nominee No. 2 – Weather Modification/Climate Engineering